Neurons are highly polarized cells extending long axons to their targets to form functional circuits. Subcellular targeting and local translation of mRNAs are critical for axon pathfinding, guidance, elongation, and survival. Our previous studies demonstrated that some small non-coding RNAs, microRNAs, have been suggested to localize in the axon and regulate translation of mRNAs, affecting axon branching and elongation (Wang et al., Cell Reports, 2015; Wang & Bao, Journal of Molecular Cell Biology, 2017). Our recent work revealed that axon-enriched lincRNA ALAE interacts with KHSRP and locally prevent translational suppression of GAP43 in axons, maintaining protein synthesis and subsequent axon elongation (Wei et al., Cell Reports, 2021), furthering suggesting the importance of noncoding RNAs during neuronal development.
N6-methyladenosine (m6A) is the most abundant internal modification found within eukaryotic mRNAs. m6A modification has been implicated in many biological processes, including RNA processing, translation, and decay. Recent studies report that m6A modification is highly enriched in the nervous system and plays crucial roles in neurogenesis, neuronal differentiation and axon guidance as well as learning and memory. Nevertheless, the roles and related mechanisms of m6A-methylated lincRNAs remain unclear during the development of nervous system.
This work entitled “m6A-modified lincRNA Dubr is required for neuronal development by stabilizing YTHDF1/3 and facilitating mRNA translation” was published online in Cell Reports on November 11, 2022. HUANG Jiansong and his colleagues, under the supervision of Drs. WANG Bin at the Guangdong Institute of Intelligent Science and Technology (GDIIST) and BAO Lan at the Institute of Biochemistry and Cell Biology, CAS Center for Excellence in Molecular Cell Science, CAS, and found that m6A-modified lincRNA Dubr interacts and stabilizes YTHDF1/3 through its m6A modification, thereby facilitating the translation of Tau and Calmodulin as well as maintaining subsequent axon elongation and neuronal migration.
In this study, researchers firstly analyze the profile of m6A-modified lncRNAs by m6A-CLIP data from mouse DRGs. Then, they identify a m6A-modified lincRNA Dubr abundantly expressed at early developmental stage of dorsal root ganglion (DRG) and cerebral cortex. Silencing Dubr impairs axon elongation of DRG neurons, and axon projection and migration of cortical neurons, whereas lacking m6A modification of Dubr fully loses its functions. Mechanically, Dubr interacts with m6A-binding proteins, YTHDF1/3 complex, through its m6A motifs to protect YTHDF1/3 from degradation via the proteasome pathway. Furthermore, Tau and Calmodulin are regulated by YTHDF1/3 and m6A-modified Dubr. Overexpression of YTHDF1/3 not only rescues the reduced Tau and Calmodulin but also restores axon elongation of DRG neurons by Dubr knockdown. This study uncovers a critical role of m6A-modified lincRNA in neuronal development by regulating the degradation of RNA-binding protein.
This work was collaborated with Drs. YANG Li, JIANG Xingyu and ZHANG Xu.
This work was funded by National Natural Science Foundation of China and Guangdong High Level Innovation Research Institute.
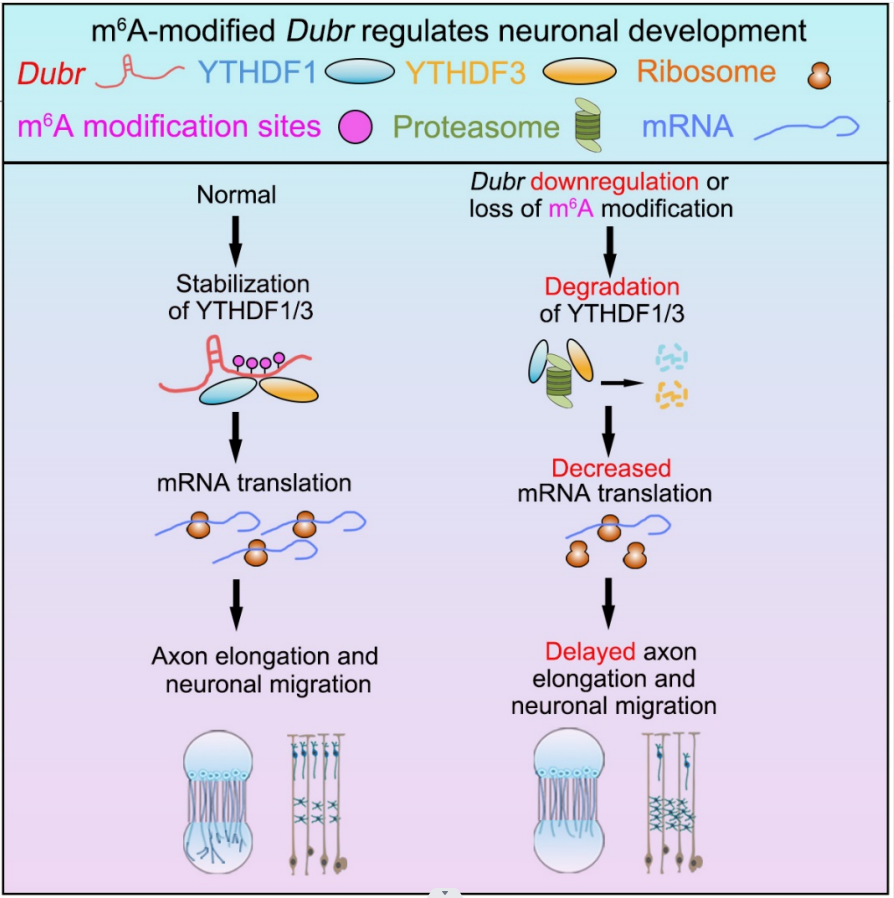
A proposed model for m6A-modified lncRNA Dubr is required for neuronal development by stabilizing YTHDF1/3 and facilitating mRNA translation
Contact: wangbin@@gdiist.cn
Reference: https://www.cell.com/cell-reports/fulltext/S2211-1247(22)01567-4


